-
silver(I) acetylide
CAS No.: 7659-31-6 Formula: C2Ag2 Molecular Weight: 239.75800
-
silver(I) acetylide
CAS No.:7659-31-6
Formula:C2Ag2
Molecular Weight: 239.75800Suppliers: All(0) China Suppliers(0) Price Available(0) Contractor(0)
Description
Pure silver acetylide is a heat- and shock-sensitive high explosive with the unusual property that on ignition it does not evolve any gas:
Ag 2C 2 (s) → 2 Ag (s) + 2 C (s)
A common misconception about the "silver acetylide" used in commercial explosives is that it explodes without the evolution of gaseous products and that its chemical formula is Ag 2C 2. In reality, it is a double salt with the silver salt it was produced from, usually silver nitrate. The anion of the parent compound acts as the oxidizer in the decomposition reaction.
The detonation velocity of the mixture silver acetylide: silver nitrate is 3460m/s. That of pure silver acetylide is 4000m/s.
Basic Info
Chemical Name |
silver(I) acetylide |
|---|---|
Synonyms |
|
CAS No. |
7659-31-6 |
Molecular Formula |
C2Ag2 |
Molecular Weight |
239.75800 |
PSA |
0.00000 |
LogP |
0.00340 |
Downstream Product
Total 5 Documents >>
Other items you might be interested in
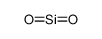
-
CAS No.: 112945-52-5
Silica, fumed
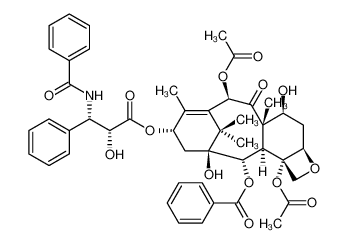
-
CAS No.: 33069-62-4
Paclitaxel
-
CAS No.: 11021-13-9
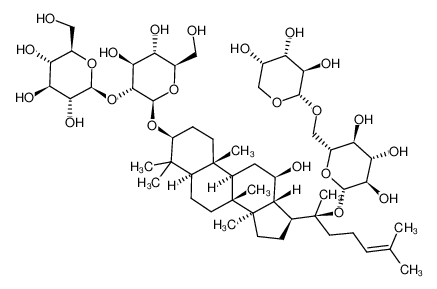
Ginsenoside Rb2
-
CAS No.: 26328-04-1
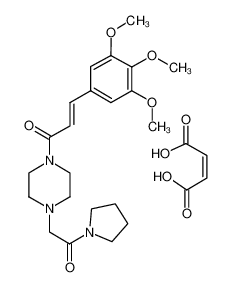
Cinepazide maleate
-
CAS No.: 501-36-0
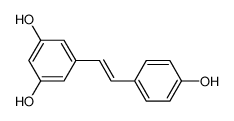
Resveratrol
-
CAS No.: 21645-51-2
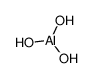
Aluminium hydroxide